Explaining Stage 2 Meaningful Use E-Health Rules
The deadlines for Stage 2 and Stage 3 have been extended by a year each.
 The U.S. government’s Centers for Medicare & Medicaid Services (CMS) last week published to the Federal Register the second of three sets of guidelines that the healthcare community must follow in rolling out electronic medical records (EMR).
The U.S. government’s Centers for Medicare & Medicaid Services (CMS) last week published to the Federal Register the second of three sets of guidelines that the healthcare community must follow in rolling out electronic medical records (EMR).
The proposed guidelines came with few surprises, but CMS did give healthcare providers an additional year to implement the changes. Those providers that ‘attest’ to meaningful use first in 2011 now have until 2014 to meet Stage 2 criteria and until 2016 to meet the Stage rules.
The proposed Stage 2 rules are now subject to a six-month comment period before they’ll be finalized.
Hospitals and private physician practices must adhere to Meaningful Use rules to qualify for Medicare and/or Medicaid EMR reimbursements, which admittedly only cover a fraction of the costs to implement the technology. The total federal cost of the Medicare and Medicaid EHR Incentive Programs is estimated to total $14.6 billion between 2014 and 2019, according to CMS.
What providers need to know
Just as with Stage 1 of Meaningful Use, Stage 2 requires healthcare providers to meet core objectives and choose from a menu of other objectives. For example, one menu option includes making up to 40% of all radiological scans available for viewing on an EMR.
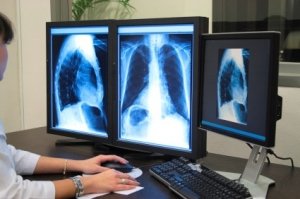 Under Stage 2 criteria, clinicians must meet (or qualify for an exclusion to) 17 core objectives and 3 of 5 menu objectives. Eligible hospitals and critical access hospitals (CAH) must meet 16 core objectives and 2 of 4 menu objectives, whereas under Stage 1 criteria clinicians were required to meet 15 core objectives and choose from 5 of 10 menu items. Clinicians must also report on 12 clinical quality measures, compared to Stage 1’s six quality measures. Eligible hospitals and CAHs must report 24 clinical quality measures, whereas Stage 1 required only 15.
Under Stage 2 criteria, clinicians must meet (or qualify for an exclusion to) 17 core objectives and 3 of 5 menu objectives. Eligible hospitals and critical access hospitals (CAH) must meet 16 core objectives and 2 of 4 menu objectives, whereas under Stage 1 criteria clinicians were required to meet 15 core objectives and choose from 5 of 10 menu items. Clinicians must also report on 12 clinical quality measures, compared to Stage 1’s six quality measures. Eligible hospitals and CAHs must report 24 clinical quality measures, whereas Stage 1 required only 15.
As expected, the proposed Stage 2 criteria focuses on how healthcare facilities can exchange key clinical information about patients and provide patients with online access to their health data.
CMS also proposed modifications to existing the Stage 1 criteria, including changes to the age limitations for vital signs, and the electronic exchange of summary-of-care documents.
Another proposed change to Stage 1 objectives includes the use of computerized physician order entry (CPOE) systems for medications. Stage 1 currently requires providers to track the number of patients receiving at least one medication. The CPOE measurement was changed so it’s a percentage of all orders, which will raise the bar considerably, according to IT healthcare consultancy CSC.
Stage 2 raises the percentage by which CPOE must be used for medications from 30% to 60%. Other new requirements include CPOE for laboratory and radiology orders.
According CSC, another important change in Stage 2 is quality measures: they are now a distinct category of meaningful use. In 2014, all those attesting to any stage of meaningful use will need to electronically report on quality measures (some may be required, others selected from a long list of potential measures). Hospitals will select 24 measures from the 50 being proposed. At least one measure will need to be reported in each area: patient safety, care coordination, population and public health, efficient use of resources and clinical effectiveness.
 Stage 2 criteria also require that radiological images and information be accessible through certified EMR technology and that cancer cases be identified and reported to a state cancer registry.
Stage 2 criteria also require that radiological images and information be accessible through certified EMR technology and that cancer cases be identified and reported to a state cancer registry.
“The Stage 2 rules are both positive and encouraging,” said Wendy Whittington, chief medical officer for Anthelio Healthcare Solutions, a Dallas-based healthcare IT service provider. “As we saw with stage 1, the details are overwhelming, but fortunately this time around, there is a sincere effort to simplify and encourage.”
Health information exchanges
The federal government has poured millions of dollars into creating health information exchanges (HIEs) that act as engines for providers to exchange patient information. The federal government is also in the process of developing a Nationwide Health Information Network. Yet the market for health information exchange is still nascent.
Dave Caldwell, executive vice president of technology integrator Certify Data Systems, said government health information exchanges will not likely be adopted by providers because they’re unwieldy. Instead, hospitals and private practices will focus private HIEs, connecting to other organizations in their region through standards, as promoted by Integrating the Healthcare Enterprise (IHE) and Health Level Seven International (HL7).
HL7 and IHE are global non-profit organizations involved in developing standards for the interoperability of health information technology. The term HL7 is also used to describe those standards based on the Open Systems Interconnection (OSI) model. IHE promotes HL7 and standards such as Digital Imaging and Communications in Medicine (DICOM), which is focused on sharing digital images between providers.
“We’ve certainly rallied around HL7 as an industry and we’re now rallying around an XML-based stream of care documents,” Caldwell said. “And we have rallied around IHE standards…and that’s what everyone is building into their infrastructure.
“The challenge is that the ambulatory EMR and EHR [software vendors] are just now starting to come out with those standards in their products,” he added. “I think you’ll see in 2012, 2013 and 2014 all of them will be adopting these standards for interoperability.”
Source: www.computerworld.com; Lucas Mearian; February 28, 2012




Leave a Reply
Want to join the discussion?Feel free to contribute!